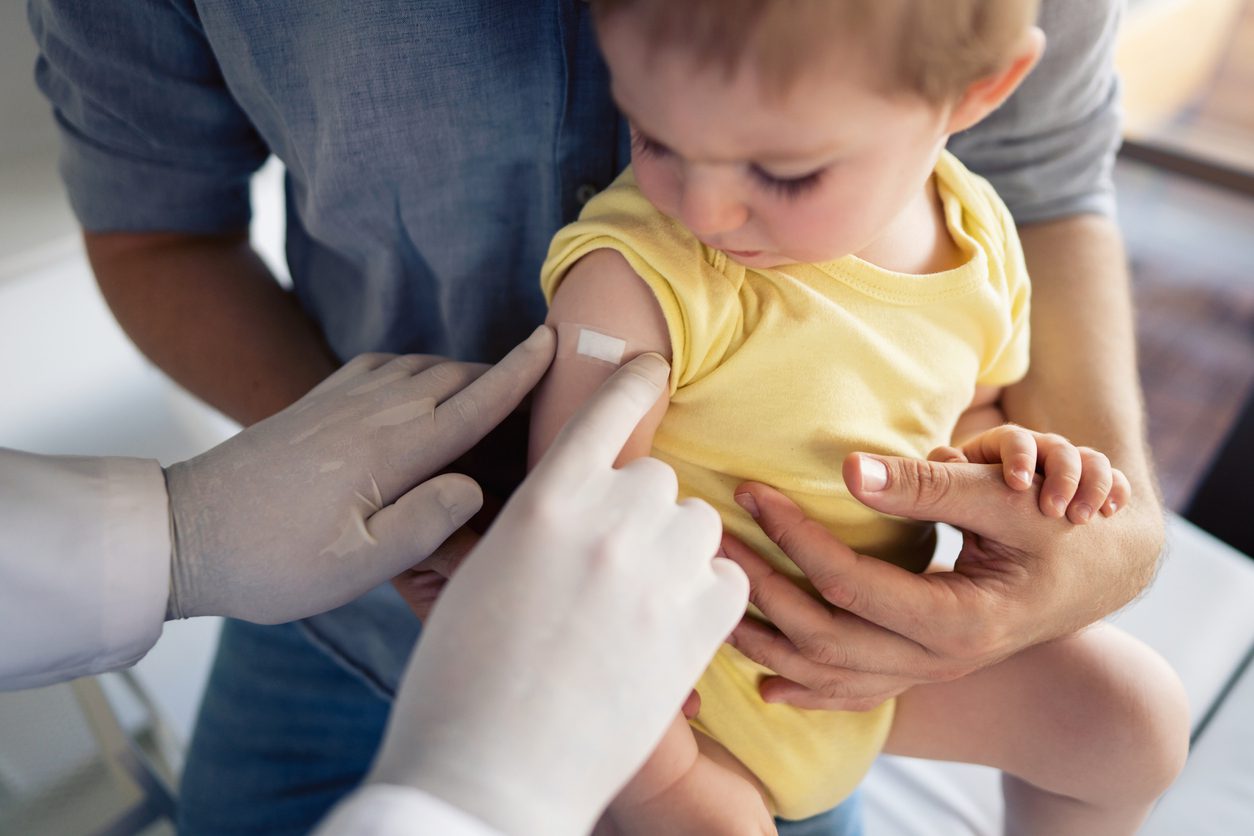
The 12-0 vote in favour of the move needs to be signed off by CDC Director Rochelle Walensky for the U.S. government to start rolling out the vaccines for children aged 5 and under.
‘We’ve taken a major step forward today,” said Oliver Brooks, Chief Medical Officer at Watts HealthCare Corporation, Los Angeles and part of the CDC Advisory Committee on Immunization Practices (ACIP).
Brooks echoed the same sentiment her fellow CDC advising member, Dr. Beth Bell, a Clinical Professor in The Department of Global Health at The University of Washington expressed after the first vote approving the Moderna shot.
“Anyone making an important decision about anything, especially for their children, want to consider that balance. Yes, we don’t know everything that there is to be known about this. Yes, the data may change. But we have a bottom line here, which is that this infection kills children and we have an opportunity to prevent that. And every parent will want to consider that calculus as well,” she said.
The U.S. Food and Drug Administration on Friday authorised Moderna Inc’s MRNA.O shot for children aged six months to 5 years, and Pfizer-BioNTech’s PFE.N, BNTX.O vaccine for children aged six months to 4 years. Pfizer’s vaccine is already authorised for children over the age of 5.