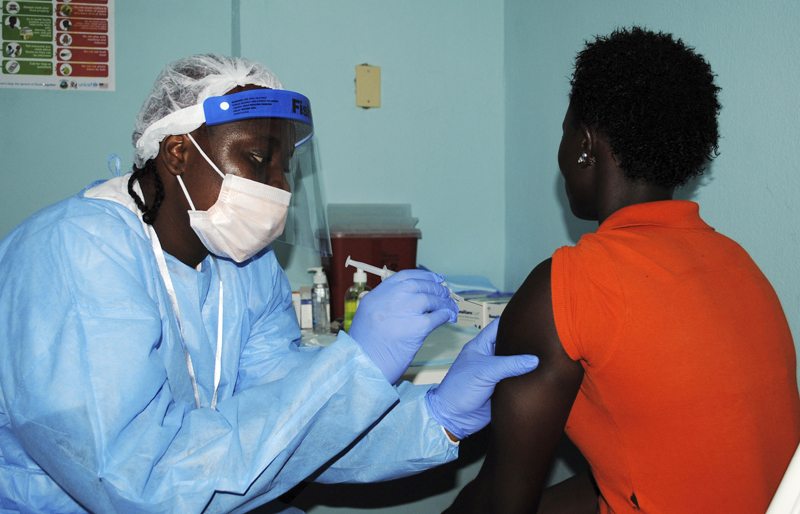
The vaccine, which contains small amounts of the virus, has been trialled on 12 volunteers and pending its response, will be administered to upwards of 27,000 more.
The vaccine aims at forcing the body to produce antibodies by administering a measured dose of the virus into willing participants.
Dr Steven Kennedy, the senior scientist at the forefront of the trials, explained that the vaccine contains a sample of the Zaire strain, which is unable to produce the Ebola virus in its participants and poses no threat to the volunteers.
As the results of the trials are yet to be identified, nurses in Liberia are being trained to monitor the reactions of participants over the coming months.
The GlaxoSmithKline vaccine is also undergoing simultaneous testing in Switzerland, UK, United States and Mali. The 200 volunteers involved in the trail have shown positive results and assured the safety of the initial candidate vaccine.
The World Health Organisation has stated the importance of committing to ongoing treatment and care; “The international response that has got us this point has been phenomenal and we must keep on course until the infection rate is brought down to, and remains at, zero.”